We used our BenAI Engine to identify a leading COVID-19 treatment, which is now FDA approved.

Rapid response
Pivoting our focus to decipher the novel coronavirus
In January 2020, as the pandemic spread, we set out to find an existing drug that could be repurposed as a COVID-19 treatment.
Although we designed our technology to develop new drugs for disease – not identify new uses for existing medications – we could make this pivot thanks to our comprehensive data foundations and flexible, disease-agnostic approach.
Novel insights
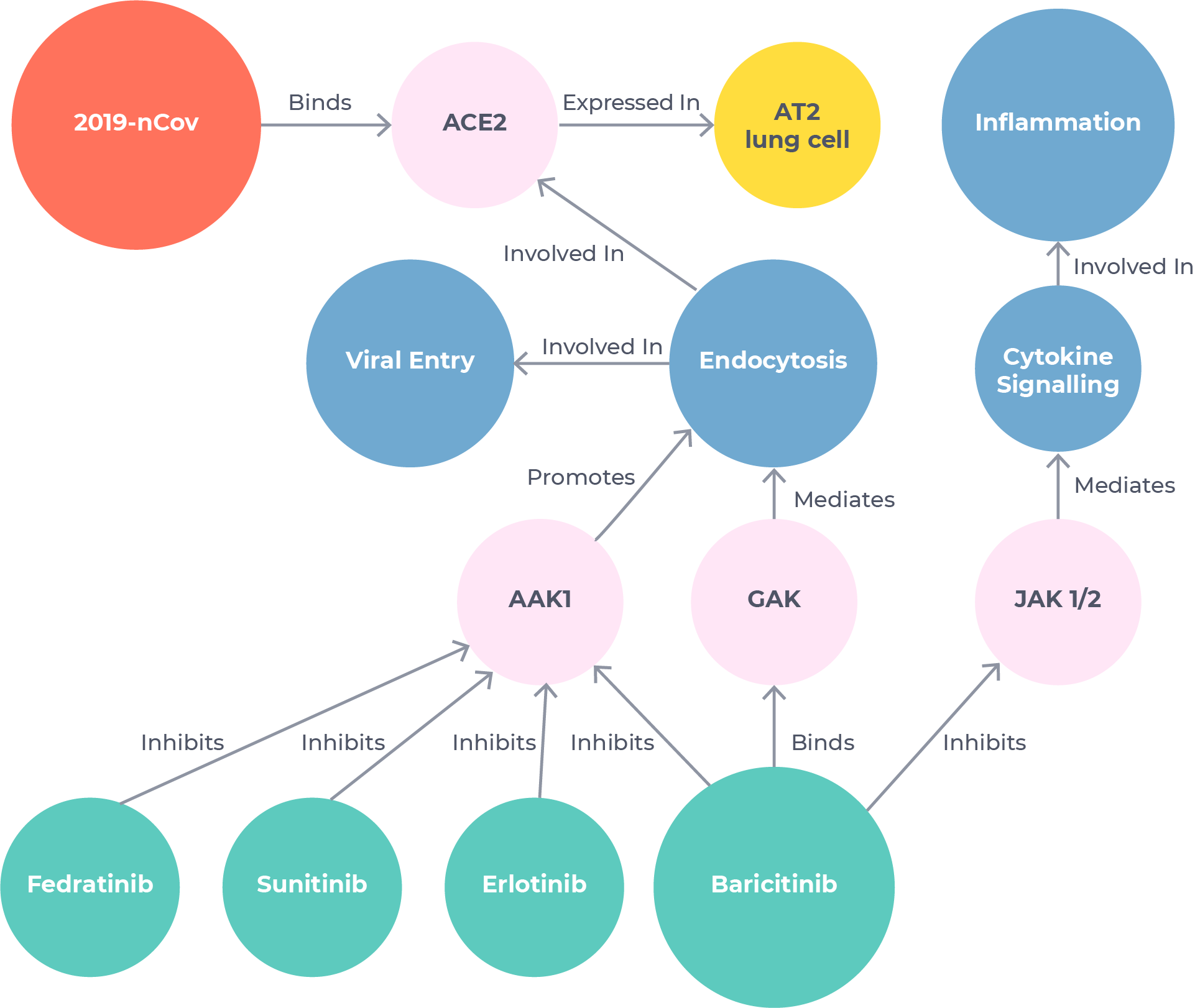
Uncovering a leading COVID-19 treatment in just 48 hours
BenevolentAI scientists used our tools to search the Knowledge Graph for mechanisms related to viral infection and inflammatory response. They identified baricitinib – a drug owned by Eli Lilly and approved for rheumatoid arthritis – as the strongest candidate.1 This hypothesis was based on novel information extracted from published literature using our Platform that baricitinib could have an off-target anti-viral effect in addition to being a well-known anti-inflammatory.
The whole process took just 48 hours.

Life-saving benefits
Repeated validation from randomised controlled trials
Data from four randomised controlled trials confirmed that baricitinib is a safe and effective treatment for severe COVID-19,2 with the COV-BARRIER trial showing that baricitinib plus standard of care reduced deaths by 38% across hospitalised adult patients3.
Both the World Health Organization4 and the FDA5 have recommended the use of baricitinib for hospitalised COVID-19 patients.
PARTNERING
We partner with leading pharmaceutical and biotech companies to help them unlock biological insights and tackle complex therapeutic challenges.
- Richardson et al. Lancet 395, e30–e31 (2020).
- Selvaraj et al. EClinicalMedicine 49, 101489 (2022).
- Marconi et al. Lancet Respir Med 9, 1407–1418 (2021).
- BMJ 370, m3379 (2020).
- Lilly Press Release (11 May 2022).
